Lifefit Tutorial¶
[1]:
# import modules
import lifefit as lf
import numpy as np
import matplotlib.pyplot as plt
import seaborn as sns
import os
# seaborn settings
sns.set_style('white')
sns.set_context("notebook")
sns.set(font='Arial')
# plot settings
def set_ticksStyle(x_size=4, y_size=4, x_dir='in', y_dir='in'):
sns.set_style('ticks', {'xtick.major.size': x_size, 'ytick.major.size': y_size, 'xtick.direction': x_dir, 'ytick.direction': y_dir})
Lifetime¶
First, define the path to the data
[23]:
atto550_dna_path = lf._DATA_DIR+'/lifetime/Atto550_DNA.txt'
irf_path = lf._DATA_DIR+'/IRF/irf.txt'
Next, we read in our datafile for the fluorescence decay and the instrument response function (IRF). Instead of using the lf.read_decay() function we can define a custom import function that outputs a two-column array containing numbered channels and intensity counts.
[24]:
atto550_dna, timestep_ns = lf.tcspc.read_decay(atto550_dna_path)
irf, _ = lf.tcspc.read_decay(irf_path)
Next we instantiate a Lifetime object by rpoviding the data arrays of the fluorescence decay and the IRF along with the timestep between two channels
[25]:
atto550_dna_life = lf.tcspc.Lifetime(atto550_dna, timestep_ns, irf)
Fit the fluorecence decay by iterative reconvolution with the IRF
[26]:
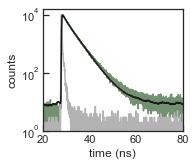
atto550_dna_life.reconvolution_fit([1,5])
=======================================
Reconvolution fit with experimental IRF
tau0: 1.01 ± 0.01 ns (29%)
tau1: 3.89 ± 0.01 ns (71%)
mean tau: 3.61 ± 0.01 ns
irf shift: 0.11 ns
offset: 1 counts
=======================================
Plot the IRF, the fluorescence decay and the fit
[27]:
with sns.axes_style('ticks'):
set_ticksStyle()
f, ax = plt.subplots(nrows=1, ncols=1, figsize=(2.5,2.25), sharex=False, sharey=True, squeeze=False)
ax[0,0].semilogy(atto550_dna_life.fluor[:,0], atto550_dna_life.fluor[:,2], color=[0.45, 0.57, 0.44])
ax[0,0].semilogy(atto550_dna_life.irf[:,0], atto550_dna_life.irf[:,2], color=[0.7,0.7,0.7])
ax[0,0].semilogy(atto550_dna_life.fluor[:,0], atto550_dna_life.fit_y, color='k')
ax[0,0].set_ylabel('counts')
ax[0,0].set_xlabel('time (ns)')
ax[0,0].set_xlim((20,80))
ax[0,0].set_ylim(bottom=1)
Anisotropy¶
Read the four different fluorescence decays and generate a lifetime object from each channel
[29]:
atto550_dna_path = {}
atto550_dna = {}
atto550_dna_life = {}
for c in ['VV','VH','HV','HH']:
atto550_dna_path[c] = lf._DATA_DIR+'/anisotropy/{}.txt'.format(c)
atto550_dna[c], fluor_nsperchan = lf.tcspc.read_decay(atto550_dna_path[c])
atto550_dna_life[c] = lf.tcspc.Lifetime(atto550_dna[c], fluor_nsperchan, irf)
Compute an anisotropy object from the lifetime objects and fit a two-rotator model to the anisotropy decay
[30]:
atto550_dna_aniso = lf.tcspc.Anisotropy(atto550_dna_life['VV'], atto550_dna_life['VH'], atto550_dna_life['HV'],atto550_dna_life['HH'])
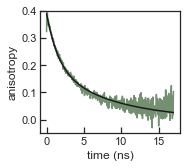
atto550_dna_aniso.rotation_fit(p0=[0.4, 1, 10,1], model='two_rotations')
====================
Anisotropy fit
model: two_rotations
r0: 0.19 ± 0.01 ns
b: 0.00 ± 0.02 ns
tau: 8.50 ± 0.45 ns
tau2: 1.57 ± 0.14 ns
====================
Plot the anisotropy decay with the fit
[31]:
with sns.axes_style('ticks'):
set_ticksStyle()
f, ax = plt.subplots(nrows=1, ncols=1, figsize=(2.5,2.25), sharex=False, sharey=True, squeeze=False)
ax[0,0].plot(atto550_dna_aniso.time, atto550_dna_aniso.r, color=[0.45, 0.57, 0.44])
ax[0,0].plot(atto550_dna_aniso.time, atto550_dna_aniso.fit_r, color='k')
ax[0,0].set_ylim((-0.05,0.4))
ax[0,0].set_xlabel('time (ns)')
ax[0,0].set_ylabel('anisotropy')